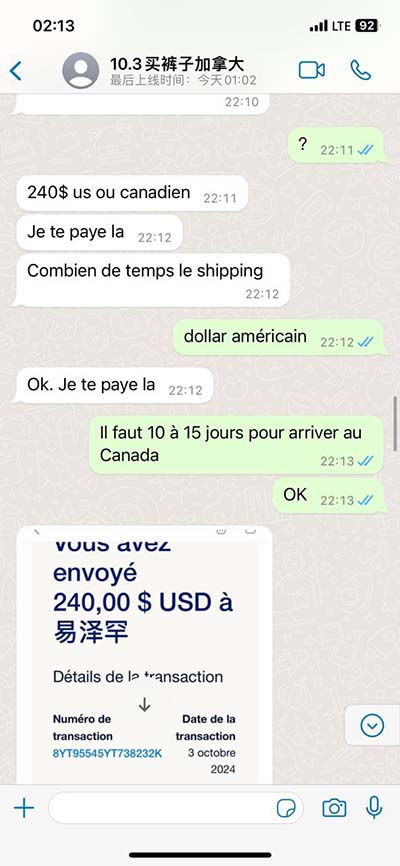
ziltivekimab hermes | ziltivekimab ziltivekimab hermes The HERMES trial (a research study to investigate how ziltivekimab works compared with placebo in people with heart failure and inflammation; NCT05636176) is assessing the effect of this novel therapeutic .
All plugs have 14mm hex - just one wrench works for all! Hi-Temp Gasket - Red hi-temp gasket for max sealing protection. Easy ID - Thread size etched on each plug. Rust Protection - Black oxide coating for rust and corrosion protection. Avoid Cross-Threading - Pilot-point tip to avoid cross-threading during installation. Specifications.
0 · ziltivekimab high sensitivity
1 · ziltivekimab antibody
2 · ziltivekimab and inflammation
3 · ziltivekimab and il 6
4 · ziltivekimab and Hermes
5 · ziltivekimab
6 · Hermes ziltivekimab side effects
7 · Hermes trial ziltivekimab
The 17th century was a period of unceasing disturbance and violent storms, no less in literature than in politics and society. The Renaissance had prepared a receptive environment essential to the dissemination of the ideas of the new science and philosophy.
The HERMES trial is an international, multicentre, parallel group, randomized, double-blind, study in patients with HFpEF and HFmrEF, evaluating the effect of ziltivekimab 15mg compared with . This study will determine if ziltivekimab, a monoclonal antibody, can be used to treat people living with heart failure and inflammation. Previous studies showed that .We would like to show you a description here but the site won’t allow us. This randomised, double-blind, phase 2 trial shows that ziltivekimab, a fully human monoclonal antibody directed against the IL-6 ligand, safely and effectively reduces .
We therefore addressed whether ziltivekimab, a fully human monoclonal antibody directed against the IL-6 ligand, safely and effectively reduces biomarkers of inflammation and . The HERMES trial (a research study to investigate how ziltivekimab works compared with placebo in people with heart failure and inflammation; NCT05636176) is assessing the effect of this novel therapeutic . In a US-based phase 2 trial, ziltivekimab, a fully human monoclonal antibody targeting the interleukin-6 ligand, significantly reduced biomarkers of inflammation compared . TLDR. The findings underscore the importance of personalized treatment strategies on heart failure, with a focus on inflammation-driven pathways, positioning anti-inflammatory therapies as a significant advancement in cardiovascular disease management. Expand.

IL-6 inhibition with ziltivekimab in patients at high atherosclerotic risk (RESCUE): a double-blind, randomised, placebo-controlled, phase 2 trial We therefore addressed whether ziltivekimab, a fully human monoclonal antibody directed against the IL-6 ligand, safely and effectively reduces biomarkers of inflammation and .The HERMES trial is an international, multicentre, parallel group, randomized, double-blind, study in patients with HFpEF and HFmrEF, evaluating the effect of ziltivekimab 15mg compared with placebo, given once a month, in addition to standard care, on the primary composite outcome of time to first occurrence of cardiovascular death, heart . This study will determine if ziltivekimab, a monoclonal antibody, can be used to treat people living with heart failure and inflammation. Previous studies showed that ziltivekimab can lower inflammation, which may have a positive effect on heart failure symptoms.
We would like to show you a description here but the site won’t allow us. This randomised, double-blind, phase 2 trial shows that ziltivekimab, a fully human monoclonal antibody directed against the IL-6 ligand, safely and effectively reduces biomarkers of inflammation and thrombosis among patients with high cardiovascular risk.
We therefore addressed whether ziltivekimab, a fully human monoclonal antibody directed against the IL-6 ligand, safely and effectively reduces biomarkers of inflammation and thrombosis among patients with high cardiovascular risk. The HERMES trial (a research study to investigate how ziltivekimab works compared with placebo in people with heart failure and inflammation; NCT05636176) is assessing the effect of this novel therapeutic monoclonal antibody targeting the IL-6 ligand, in people living with HFpEF and who have concomitant inflammation. In a US-based phase 2 trial, ziltivekimab, a fully human monoclonal antibody targeting the interleukin-6 ligand, significantly reduced biomarkers of inflammation compared with placebo in patients at high atherosclerotic risk. Here, we report the efficacy and safety of ziltivekimab in Japanese patients. TLDR. The findings underscore the importance of personalized treatment strategies on heart failure, with a focus on inflammation-driven pathways, positioning anti-inflammatory therapies as a significant advancement in cardiovascular disease management. Expand.
IL-6 inhibition with ziltivekimab in patients at high atherosclerotic risk (RESCUE): a double-blind, randomised, placebo-controlled, phase 2 trialThis study aims to determine if ziltivekimab can be used to treat people living with heart failure and inflammation in reducing the risk of cardiovascular death and heart failure events. Participants will receive either ziltivekimab or placebo (a pill with no active drug ingredient).The HERMES trial is an international, multicentre, parallel group, randomized, double-blind, study in patients with HFpEF and HFmrEF, evaluating the effect of ziltivekimab 15mg compared with placebo, given once a month, in addition to standard care, on the primary composite outcome of time to first occurrence of cardiovascular death, heart .
This study will determine if ziltivekimab, a monoclonal antibody, can be used to treat people living with heart failure and inflammation. Previous studies showed that ziltivekimab can lower inflammation, which may have a positive effect on heart failure symptoms.We would like to show you a description here but the site won’t allow us. This randomised, double-blind, phase 2 trial shows that ziltivekimab, a fully human monoclonal antibody directed against the IL-6 ligand, safely and effectively reduces biomarkers of inflammation and thrombosis among patients with high cardiovascular risk.
We therefore addressed whether ziltivekimab, a fully human monoclonal antibody directed against the IL-6 ligand, safely and effectively reduces biomarkers of inflammation and thrombosis among patients with high cardiovascular risk. The HERMES trial (a research study to investigate how ziltivekimab works compared with placebo in people with heart failure and inflammation; NCT05636176) is assessing the effect of this novel therapeutic monoclonal antibody targeting the IL-6 ligand, in people living with HFpEF and who have concomitant inflammation. In a US-based phase 2 trial, ziltivekimab, a fully human monoclonal antibody targeting the interleukin-6 ligand, significantly reduced biomarkers of inflammation compared with placebo in patients at high atherosclerotic risk. Here, we report the efficacy and safety of ziltivekimab in Japanese patients. TLDR. The findings underscore the importance of personalized treatment strategies on heart failure, with a focus on inflammation-driven pathways, positioning anti-inflammatory therapies as a significant advancement in cardiovascular disease management. Expand.
rolex gmt hands
IL-6 inhibition with ziltivekimab in patients at high atherosclerotic risk (RESCUE): a double-blind, randomised, placebo-controlled, phase 2 trial
ziltivekimab high sensitivity
ziltivekimab antibody
ziltivekimab and inflammation

The best books published during the 17th century (January 1st, 1601 through December 31st 1700). See also Most Rated Book By Year Best Books By Century: 21st, 20th, 19th, 18th, 17th, 16th, 15th,14th, 13th, 12th, 11th, 10th, 9th, 8th, 7th, 6th, 5th, 4th Lists for all books by Number of Ratings:
ziltivekimab hermes|ziltivekimab